
Decayment of locomotor activity is a robust readout for detecting early onset of aging. It allows the easy discovery of new genes and the search for compounds to reverse aging. In this experiment we show the application of ARENA to measure activity of worms in 24well plates using liquid medium, and we present the full protocol for motility based lifespan research.
MATERIALS:
-24 well plate with lid.
– Synchronized L4 worms (N2 strain).
– S Complete Medium (See recipe below)
– Streptomycin stock 100 mg/ml.
– Kanamycin stock 100 mg/ml.
– Amphotericin B stock 250 µg/ml.
– Fluorodeoxyuridine (FUDR) 100x stock
– OP50 feeding bacteria in S-complete media 100 mg/ml.
– WMicrotracker ARENA device.
METHOD:
Sterile conditions are need by using flow cabinet or flame during manipulation of the worms. Perform at least four technical replicates and at least two biological replicates.To prevent starvation of worms, feeding bacteria need to be added. You might have to add bacteria in day 3, 7, 12, 19, 26.
- After recording the activity of the worms using wMicrotracker ARENA, a fast inspection of the plate is recommended to assess if bacteria need to be added.
- Remove the sealer from the micro-plate maintaining sterile conditions.
- Add 20 μL of 10 mg/ml bacterial suspension in each well and seal the plate again.
- Shake the culture gently by hand.
- Return it into the incubator.
DAY -1_ Transfer L4 worms to NGM+FUdR Plate
- Transfer your Synchronized L4 worms to another NGM+FUdR plate containing E. coli. [NOTE: this pretreatment in NGM increase the efficacy of FUdR to avoid worm reproduction]
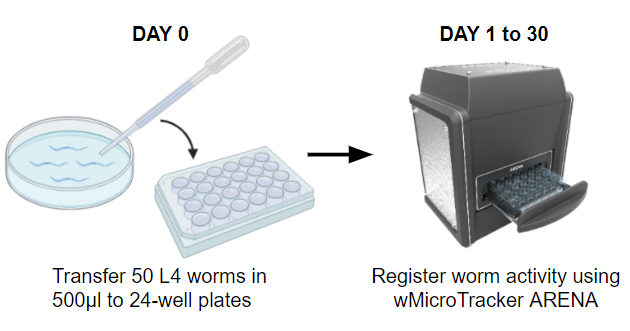
DAY 0_ Transfer young adult worms to 24-well plate
- Collect Synchronized Young adult worms by washing the NGM agar plates with S Complete medium.
- Adjust the final volume so that worm concentration in the suspension is approximately 100 worms/ml.
- Add streptomycin (to a final concentration 100 μg/mL), kanamycin (20 μg/mL) and the antifungal drug Amphotericin B (0,1 μg/mL) to avoid contamination.
- Add an appropriate volume of feeding bacteria in S-complete media to a final concentration of 3 mg/mL.
- Add FUdR to a final concentration of 50 μM to 120 μM [NOTE: a pre-setup is needed].
- Mix the worm suspension. Transfer 500 μL of the suspension in each well of a 24-well plate (final: 50 worms/well).
- Seal the plate to avoid contamination.
- Put the plate in the incubator at the desired temperature.
DAY 1 to 50. _ Measure locomotor activity once a day.
- Before setting the plate into the wMicrotracker ARENA, make sure there is no condensed water on the plate lid. If necessary, dry the plate lid maintaining sterile conditions.
- Shake the microplate of worms gently by hand or stimulate it using high intensity blue light to induce animal movement.
- Record the activity of the plate with worms using wMicrotracker ARENA for 30 min at the same temperature of the incubator.
- Generate the data report using the software.
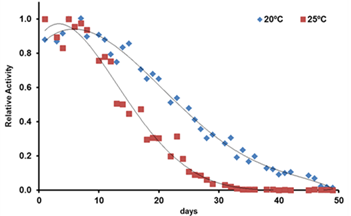
Locomotion-based healthspan assay
Locomotion activity of N2 was evaluated during 50 days. On day 0, Young adult stage N2 worms were transferred to S Complete Medium with 50 µM FudR in a 24-well plate. The worms were allowed to grow at 20ºC or 25ºC. Locomotor activity was recorded at 20ºC/25ºC during 30 min. Data represents the mean activity recorded each day (n=9 per group, 2 independent experiments)
Additional RECIPES (from Ian Hope book)
– S-basal medium:
For 1 L, add:
5.9 g NaCl,
50 mL of 1 M Potassium Phosphate Buffer pH 6.0
Adjust the volume with ddH2O up to 1 L and autoclave.
Let the medium cool down and add 1 mL of 5 mg/mL cholesterol (dissolved in ethanol).
– S-complete medium:
For 1 L, add:
977 mL S-basal medium,
10 mL 1 M Potassium citrate pH 6 (sterile)
10 mL Trace metal solution (sterile)
3 mL 1 M CaCl 2 (sterile)
3 mL 1 M MgSO 4 (sterile).
Use sterile technique, do not autoclave.
