
Over the past decade, major advances in phenotypic screening have produced automated, quantitative motility and development assays for nematodes (e.g., Caenorhabditis elegans and Haemonchus contortus) and trematodes (e.g., Schistosoma mansoni) using platforms such as the WMicroTracker system — an automated system that quantifies the movement of small animals over time and space utilising infrared technology (Gunderson et al., 2020). This assay has enabled medium- to high-throughput and reproducible evaluation of compound activity and potency using larval parasite stages. In contrast, equivalent platforms have not been developed for F. hepatica or other liver flukes. The free-living miracidium stage of F. hepatica provides an attractive opportunity to address this gap.
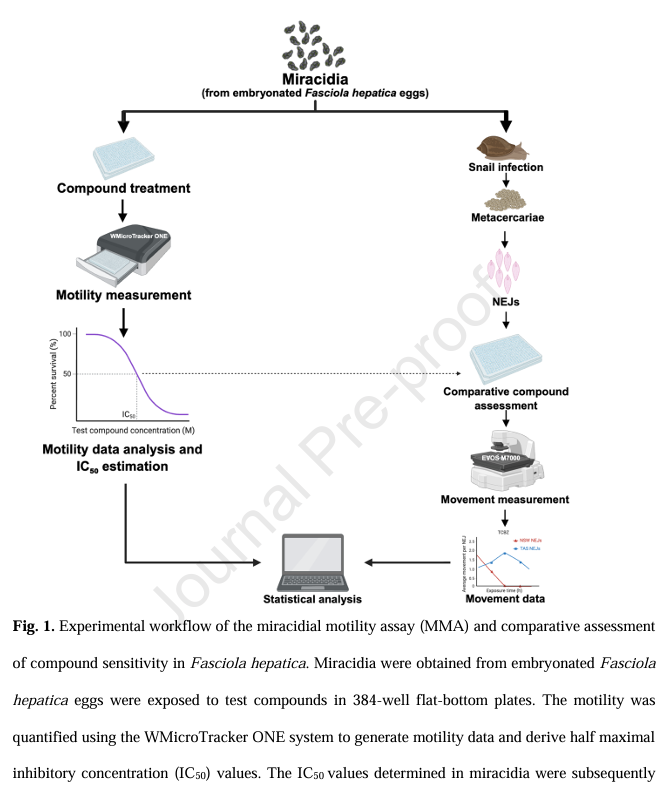
Fasciola hepatica causes fasciolosis in livestock and humans worldwide, yet reliable tools to assess drug efficacy against the early developmental stages of this parasite are lacking. Here, we developed an automated miracidial motility assay (MMA) using the WMicroTracker ONE infrared detection system to quantify the sensitivity of F. hepatica miracidia to anthelmintic compounds including clorsulon (CLORS), closantel (CLOS), triclabendazole (TCBZ) and triclabendazole-sulphoxide (TCBZ-SO).
Methods
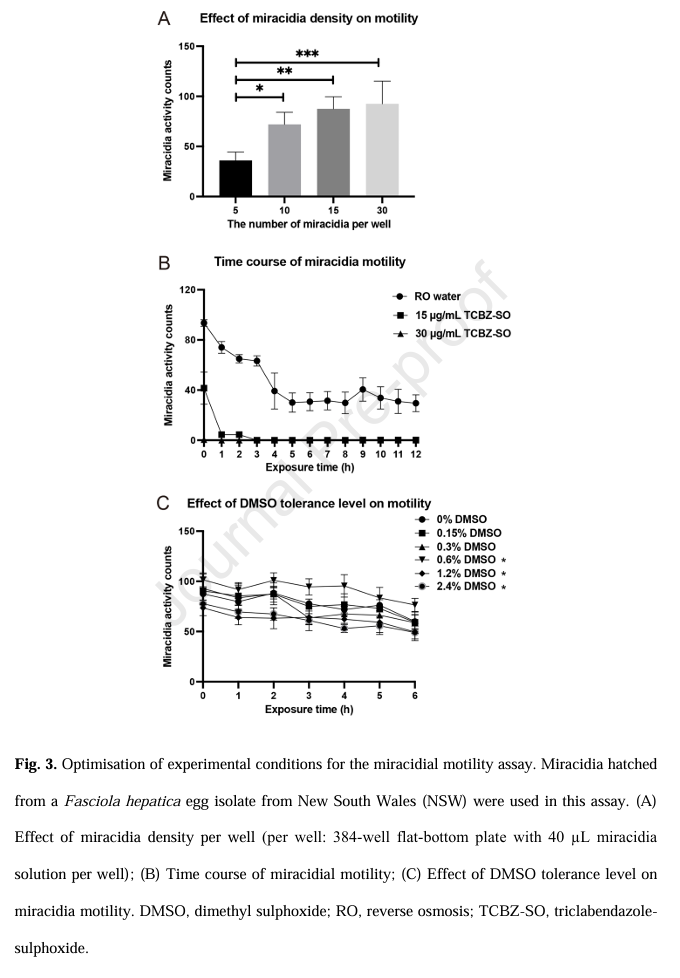
Optimisation of the miracidial motility assay (MMA). To establish a robust phenotypic assay suitable for medium- to high-throughput screening, experimental conditions were first optimised using the F. hepatica isolate from NSW. First, to establish the suitable miracidial density range in 384-well flat-bottom microplates, four densities (5, 10, 15 and 30 miracidia per well) were assessed, each in quadruplicate (n = 4), using the WMicroTracker ONE system. Second, the time of the motility measurement was optimised in a 384-well flat-bottom plate, with 15 miracidia per well and four technical replicates (n = 4). Blank control-wells contained miracidia and sterile RO water, whereas test-wells contained miracidia plus 15 or 30 µg/mL TCBZ SO prepared in 0.15% or 0.3% DMSO, respectively. Third, the optimal measurement window that showed the greatest difference in motility between the control and test groups was established. Fourth, to determine the maximum DMSO concentration that did not significantly impair motility, six DMSO concentrations (0%, 0.15%, 0.3%, 0.6%, 1.2% and 2.4%) were each tested in quadruplicate using 15 miracidia per well in a 384-well plate.
Results
Here, we established a phenotypic assay (MMA) in a 384-well plate format for the quantitative assessment of the sensitivity of Fasciola hepatica miracidia to anthelmintic drugs. Through systematic optimisation of this assay, we identified an optimum inoculum of 10–15 miracidia per well, a three-hour observation window and a solvent (DMSO) threshold of 0.6% as critical parameters for reliable assay performance.The experimental workflow is depicted in Fig.1. Miracidia were obtained from embryonated F. hepatica eggs, which were collected from the gallbladders of naturally infected sheep. Newly hatched miracidia were utilised directly for the optimisation (Fig 3) and application of a miracidial motility assay (MMA) to quantitatively assess compound sensitivity. WMicroTracker platform has the potential to be a versatile and scalable tool for investigations of drug susceptibility and resistance across a broader range of helminth parasites.
International Journal for Parasitology: Drugs and Drug Resistance, 2026.
Mengwei Zheng, Aya C. Taki, Tanapan Sukee, Jane Hodgkinson, Terry W. Spithill, Robin B. Gasser, Neil D. Young.
