
Cannabidiol (CBD), the second most abundant phytocannabinoid in Cannabis sativa, has garnered significant interest due to its non-psychoactive nature and diverse receptor interactions. This study employs in vitro and in vivo methodologies to validate CBD’s potential as a treatment for Alzheimer’s disease (AD) by addressing key hallmarks of the condition and promoting neuroprotective effects on spatial memory.
Methods
C. elegans motility assay. For the CBD effects, a suspension of the L1 larval stage, containing 25–35 larvae, was incubated with 20 μL of inactivated E. coli and 25 μL of different CBD concentrations (10 μM and 100 μM) in a 96-wellplate. The movements of the worms were then meticulously recorded for 20 min using the WMicroTracker MINI (Phylumtech S.A., Argentina). The travel distance and movement speed were further evaluated using theWMicrotracker SMART equipment (Phylumtech S.A).
Results
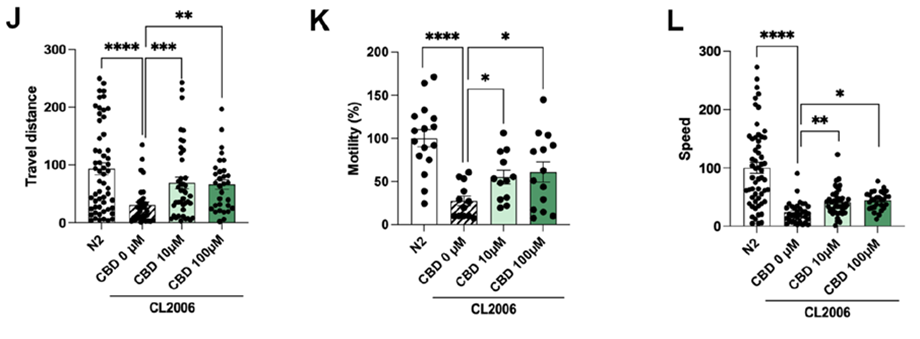
Cognitive improvement after CBD treatment in the in vivo animal models C. elegans. To evaluate the impact of CBD on the progression of AD, the CL2006 strain, one of the best characterized transgenic AD strains, was employed. This strain contains the Punc-54::Aβ1–42 transgene, which results in progressive adult-onset paralysis, that has been correlated with increased levels of Aβ aggregates. As shown in Fig. 6J, treatment with CBD at 10 μM and 100 μM resulted in improved motility of CL2006 worms compared to the CL2006 control group. Accordingly, CBD treatment significantly increased travel distance and speed (Fig. 6K, L), confirming the delay of the progressive adult-onset paralysis in CL2006. We also measured the Aβ deposits in the head of the C. elegans strain CL2006, demonstrating that CBD treatment at both 10 μM and 100 μM concentrations shows efficacy to reduce the degree of Aβ oligomerization.
Alzheimers Res Ther. 2025 May.
Raïch I, Lillo J, Rebassa JB, Griñán-Ferré C, Bellver-Sanchis A, Reyes-Resina I, Franco R, Pallàs M, Navarro G.
