
Root lesion nematodes (RLNs) are major plant parasites causing significant global yield losses in a wide range of crops. Current management strategies largely depend on synthetic nematicides, which raise environmental and human health concerns due to their broad-spectrum toxicity and persistence in the ecosystem. Volatile allelochemicals offer a promising, environmentally safer alternative due to their biodegradability and lower toxicity to mammals. In this study, we assessed the nematicidal activity of five allele chemical volatiles—dimethyl sulphide (DMS), dimethyl disulphide (DMDS), trans-cinnamaldehyde(TCA), trans-2-decenal (T2D), and trans-2-undecenal (T2U)—against Pratylenchus penetrans, using direct-contact bioassays, in comparison with the conventional nematicide oxamyl.
Methods
Determination of Motility Inhibition Through Direct Contact Bioassays. Inhibition of RLN (Pratylenchus penetrans) motility was assessed with a WMicrotracker MINI device (PhylumtechS.A., Santa Fe, Argentina), which can quantify nematode movement through the scattering of infrared light. Movement was detected through the interference of a light beam passing through the aqueous suspension containing the RLNs, in each well of a microtiter plate. Motility was quantified as the number of continuous interferences, during a selected time interval. The experimental setup followed the description in the previous section with modifications. Briefly, 38 μL of a suspension with 25 to 35 mixed life stage RLNs was pipetted to each well of a U-bottom 96-well microtiter plate and 2 μL of compound stock solution (at 20 mg/mL) was added to obtain a final compound concentration of 1 mg/mL. The microplate was covered and mixed in an orbital shaker at 800 rpm for 1 min. In each microtiter plate, blank wells were performed to assess control RLN motility by adding 2 μL of ultrapure water instead of the stock solution and control wells were added with 2 μL of methanol to determine the contribution of methanol to RLN motility inhibition. Nematode movement in each well was quantified, for 60 min, after incubation at 25 ± 1 ◦C for 24, 48 or 72 h. For each treatment, three independent experiments were performed, each with at least eight replicates.
Results
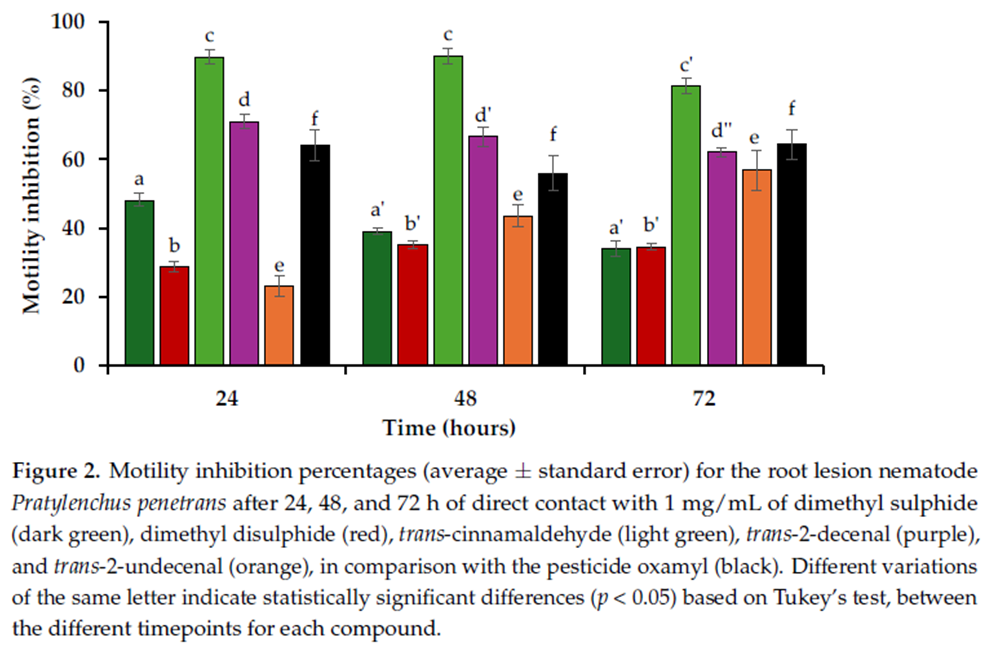
Nematotoxic Activity. The inhibition of P. penetrans motility was assessed by quantifying nematode movement after 24, 48, and 72 h in direct contact with 1 mg/mL of the tested allelochemicals. The organosulfur compounds demonstrated variable levels of motility inhibition. DMS exhibited relatively low inhibition values, with a slight decrease over time, from 48.2 ± 1.9% at 24 h to 39.1 ± 1.0% at 48 h and 34.0 ± 2.2% at 72 h (Figure 2). The motility inhibition activity of DMDS can also be described as low or inactive, with values remaining consistently below the 40% motility inhibition, increasing from 28.7 ± 1.5% at 24 h to 35.2 ± 1.1% at 48 h, followed by a slight decrease to 34.5 ± 1.0% at 72 h (Figure 2).
The phenylpropanoid TCA showed high motility inhibition throughout the 72 h period, achieving 89.7 ± 1.3% after 24 h, with a slight increase over time to 90.1 ± 2.1% at 48 h and a decrease to 81.4 ± 2.2% at 72 h (Figure 2). The tested aldehydes exhibited a contrasting trend. While T2U demonstrated low initial inhibition which increased over time, from 23.1 ± 2.2% at 24 h to 43.6 ± 2.8% at 48 h and 56.8 ± 1.4% after 72 h, T2D showed greater activity, with inhibition values decreasing over time from 71.0 ± 3.1% at 24 h to 66.6 ± 3.2% at 48 h and 62.0 ± 5.8% at 72 h (Figure 2). The synthetic nematicide oxamyl exhibited moderate motility inhibition, with no significant change in activity over the course of the 72 h, with motility inhibition ranging from 64.1 ± 4.4% at 24 h to 56.0 ± 4.9% at 48 h, before increasing to 64.3 ± 4.3% at 72 h (Figure 2).
Pereira G, Barbosa P, Vicente CSL, Faria JMS.
