Specifications
How does it works?
WMicrotracker ARENA technology is based on detection of worm movement through infrared light scattering, a methodology originally published by Simonetta SH et al 2007 (DOI: 10.1016/j.jneumeth.2006.11.015), plus a technological improvement called “temporal light interferometry detection” (Phylumtech SA, Argentinean Patent 2019 #W576818 “Procedure and device for automatic recording of nematode locomotion or small organisms of similar sizes by temporal interferometry of light microbeams”)
This system possess a sensing arena composed of more than 20.000 infrared microbeams, 100um wide, illuminating the microplate from the bottom side. The light beams generated by infrared leds are separated 0.7mm one each other.
An optical system of 6 video cameras (1Mpixel each) capture the light beams from the top side and the analog signal is mathematically processed to detect the movement of the small animals in the sensing arena.
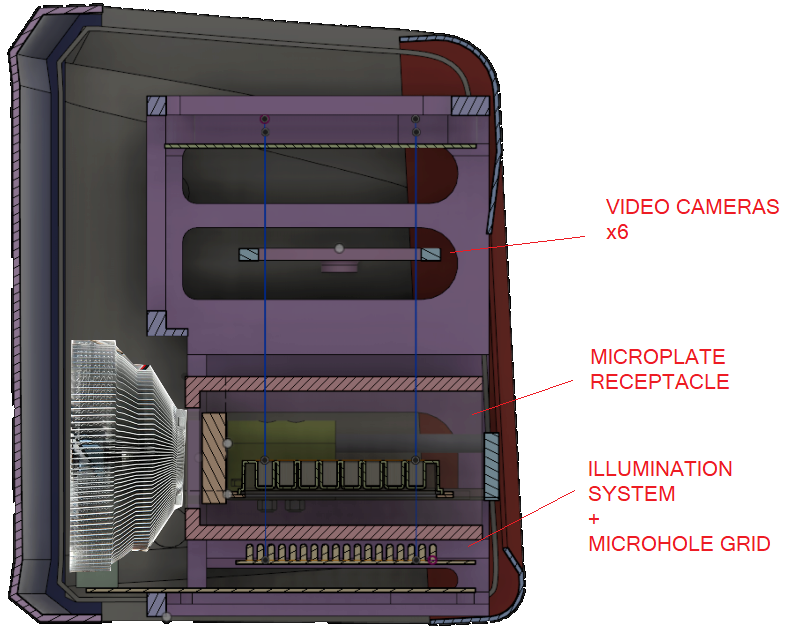
How to position the microplate in the tray?
Place the microplate with the “A1” well positioned at the top left, as shown in the following picture.
How fast is the data acquired?
Arena acquisition mode will multiplex the microplate in groups of wells, 1 row of wells per camera at time. The multiplexing mode will take 10 pictures per row, with a maximum rate of 1frame/second, according to each microplate format. This mode allows to optimize the detection of fast moving small animals in any culture medium (liquid or solid).
How is the data processed?
The software will acquire the signal of each microbeam and will process it mathematically to detect small animals light scattering. If movement is detected, an activity counter will be incremented.
The total calculated activity will be the integration of activity events in a time frame defined by the user.
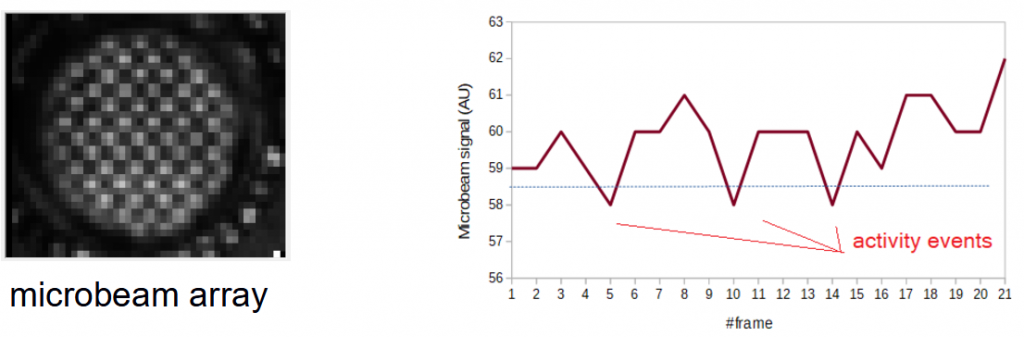
Example of capture of one microbeam in a well:
Example of worm population activity detected in 35mm Petri dish with NGM agar:
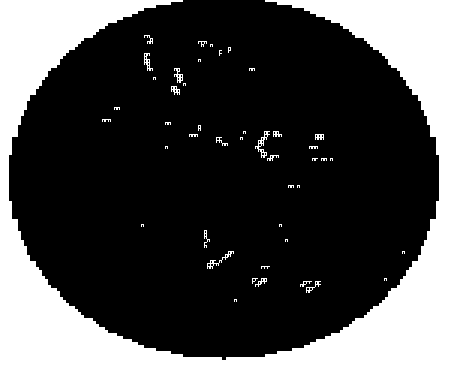
Using this approach is possible to evaluate multiple protocols using NGM cultured animals, and even liquid. Preferred microplate culture format for ARENA is 6well, 24well microplates and 35mm Petri dishes.
What is the beam size relative to the worms?
The LED beams have a diameter of 150 µm, which is larger than the diameter of an adult C. elegans worm (100 µm on average).
What are the beam specs?
- Wavelength: Infrared, 880nm
- Temperature: no heat generated
- Led technology
How many beams cross through each well?
24 well plate: 400 microbeams per well aprox.
6 well plate and 35mm Petri dish: 1950 microbeams per well aprox
IR Microbeams are separated 0.7mm one each other.
Do worms develop an aversion to the beam over time?
The infra-red beams in the wMicrotracker are generated by low-power LEDs and have been shown to be non-invasive for C. elegans.
Can I control/change the temperature of my samples with the ARENA?
Yes, the temperature can be controlled from 20°C to 37°C, +-1°C . Room temperature should be within the range of desired temperature +- 5°C for proper regulation.
Can I put the ARENA inside an incubator?
It is not recommended because optical components can be blurred. It is out of specifications.
However, we have some information about users employing: Hypoxia chambers, 4°C cold room, and 40°C bacteria incubators.
Do the beams maintain the same properties overtime?
We observed no degradation of the LEDs over time. The LEDs in the ARENA have a lifetime of 36,000 hours. Since they are working ⅙ of time at 50% 1KHz cycle, this is equivalent to more than 10 years of continuous use.
The Microtracker was engineered to auto-compensate and auto-calibrate the beams to maintain signal linearity over time.
What is the larval stage to get accurate measurements?
L4 and adults can be measured reproducible.
What is the recommended medium to get accurate measurements?
agar or liquid medium can be employed. absorbance of the medium in the infrared wavelength will decrease the detection level when OD600>1.0
How many worms should I put in each well to get accurate measurements?
For L4 and older worms, we recommend a minimum of 35 / 50 worms depending on the experimental protocol.
Signal detection linearity range is:
from 10 to 300worms (6 well plates and 35mm Petri dish)
from 5 to 100worms (24well plates).
Is the ARENA compatible with any worm strain?
The system is compatible with most worm strains including wild nematode isolates and parasitic nematodes.
We have not yet enough information to validate non motile/non-good swimming strains (such as roll or severe unc mutants).
Experimental set-up
Which type of plates can I use with ARENA?
6well microplates, 24well microplates and 35mm Petri dishes.
Can I use liquid bacteria in the well and still get accurate measurements of movement?
Yes. Worms can be measured in the ARENA for multiple days when placed in bacteria. We recommend that you use fresh bacteria at an OD600 of 0.5 (up to 1). Under these conditions, adult worms usually have enough food for 3-5 days. Another option is to cultivate your worms in axenic media (CeMM) instead of using bacteria.
Can I use NGM with bacteria in the well and still get accurate measurements of movement?
Yes. Worms can be measured in the ARENA for multiple days when placed in NGM + OP50 E. coli bacteria. Since bacteria patina is thin, it will not affect the detection. NOTE OF CAUTION: Non-OP50 bacteria, such as natural microbiota, can affect detection sensitivity if the patina is dark or wide. Perform appropriate controls before changing bacteria or consider transferring to plates without bacteria for quantification.
How long can I keep and measure my worms in the ARENA?
The ARENA can acquire data on your worms for weeks without interruption.The limiting factor for longitudinal studies will be determined by your worm culture protocol and requirements. For long term data acquisition in the ARENA, we recommend that you place your worms in axenic media, or NGM plates.
What type of liquid media can I use?
24 and 6-wells experiments work with: liquid culture of E. coli OP50, CeMM, CeHR (with or without 10% skim milk). Which one to use will depend on your preference and your experimental requirements.
Note for lifespan experiments: it has been reported that liquid culture extend lifespan compared to NGM.
Do I need to count the worms in each well?
How do I get good results if I can’t pipet precise number of worms in each well?
Pipetting worms is not as precise as dispensing same number of worms with a cell sorter or even manually. However, you can easily reduce the variability of your data by relativizing mathematically each group of wells to time 0 (referred to as “BASAL measure” in Simonetta et al.; 2007).
What are the computer requirements to use the ARENA?
Computer minimum requirements:
– Intel Core i3 / AMD 4 Core or
above (>1.2GHz clock)
– 2Gb of RAM memory
– 1 free USB
port 2.0 or 3.0
– Windows 7, 8, 10 (or higher) operating
system
– 500Mb of free HD space (>10Gb free HD space
recommended for real time data saving)
