Exploring Alpha-Synuclein and Behavioral Changes in the C. elegans Model
In the dynamic landscape of neuroscience, the quest to unravel the mysteries of neurodegenerative diseases hinges upon understanding the intricate relationship between molecular mechanisms and behavior. At the forefront of this endeavor stands alpha-synuclein, a protein whose aggregation within neurons is a defining characteristic of Parkinson’s disease and related disorders.
Recognizing alpha-synuclein as a promising therapeutic target, many researchers have turned to the C. elegans model to conduct screening assays, leveraging its genetic tractability and simplicity. The versatility of this tiny nematode allows for rapid and cost-effective exploration of the impact of alpha-synuclein on behavior, providing valuable insights into the pathophysiology of neurodegeneration.
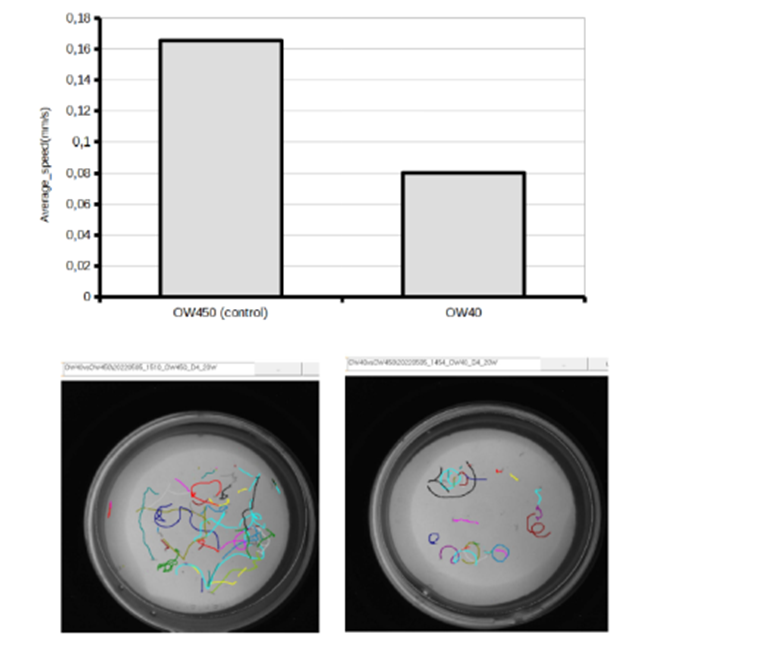
In this protocol using WMicrotracker SMART, we outline a method for studying the impact of alpha-synuclein on C. elegans behavior in a high-throughput manner. We utilized the strain OW40, which expresses human wild-type α-synuclein-YFP (α-syn) under the control of the unc-54 promoter to drive expression in body wall muscle cells, and OW450 as a control. Through the use of the WMicrotracker SMART system, it became evident that worms expressing the alpha-synuclein protein experience a gradual decrease in locomotion as the protein accumulates over time.
PROTOCOL:
WHAT YOU NEED:
100mm NGM-OP50 seeded plates
Gravid adult’s worms: OW40 (zgIs15 [P(unc54): syn:YFP]IV) and OW450 (rmIs126 [P(unc-54)Q0:YFP]V)
35 mm plates NGM plates
100 mM CuSO4 solution
M9 Buffer
Incubator
Mini Shaker
Wmicrotracker SMART
PROTOCOL
Day 1: Prepare age-synchronous cohorts by hypochlorite treatment of each worm strain: OW40 and OW450. Wash the eggs in M9 buffer 4-6 times. Place the eggs in 3 ml of M9 in a p35 dish with agitation (150 rpm) at 20°C.
Day 2 – Transfer L1 worms to NGM-OP50 seeded plates. We recommend transferring a maximum of 5000 worms per p100 plate to avoid overcrowding. Incubate at 20°C.
Days 5, 6, and 7: Transfer adult worms to new plates, making two washing steps to remove larvae. Incubate at 20°C.
Day 8: Day 4 of adulthood, transfer 20 worms to a 35mm NGM plate without food.
- Prepare plates at least one day before image acquisition; freshly prepared plates may have excess humidity that affects worm detection.
- Before adding the worms to each plate 35 mm NGM plate, create a ring of 100 mM copper sulphate around the edge of each plate to prevent worms from crawling out of the agar.
- When adding the worms, be careful not to scratch the agar, as worms tend to crawl into any breaks in the agar surface.
SMART ACQUISITION
- Open SMART Software and adjust the focus of the plate.
- Immediately before acquisition, stimulate the plate by tapping it lightly on the bench.
- Acquire data for 5 minutes using the WMicrotracker SMART system for each plate.
Plotting and analyzing the data:
In the “Check Results” menu, select the assay project. Use shift-click to select all acquisitions simultaneously, then right-click and choose the “Full Joint Report” option. This will generate a joint report containing the parameters of all acquisitions.
Create a column graph to visualize the average speed of each strain.
