
The purpose of this study was to determine the utility of the wMicroTracker as a screening platform to assess the motility of various parasites. We tested three species of parasites: the adult and larval stages of the filarial nematode Brugia pahangi, the schistosomula stage of the trematode Schistosoma mansoni, and the epimastigote stage of the protozoan parasite Trypanosoma cruzi. We optimized the assay for the number of parasites per well, plate type and media volume using the wMicroTracker and compared those readouts to readouts from the WormAssay when possible. The WormAssay has been used in phenotypic drug screens to identify new compounds for the treatment of lymphatic filariasis, onchocerciasis and schistosomiasis.
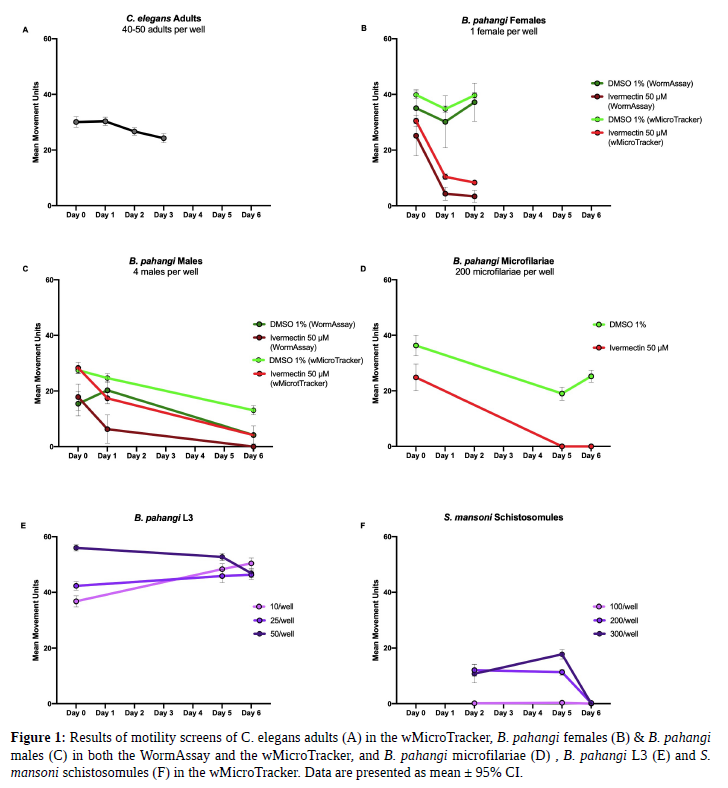
To optimize screening methods in the wMicroTracker for other parasites, the size and motility of parasites of interest were compared to C. elegans adults (40-50 adults per well in 100 μL of M9 buffer) to ensure control wells gave mean movement units around this range. B. pahangi females (34.7 mm in length by 139 μm in width ( highly motile) were screened with one worm per well in 500 μL of RPMI in a 24-well flat bottom plate with 8-9 replicate wells. B. pahangi males (18.0 mmin length by 77 μm in width, high motility) were screened with four worms per well in 500 μL ofRPMI in a 24-well flat bottom plate with 4-7 replicate wells. B. pahangi females and males were assessed in both screening platforms. B. pahangi microfilariae (mf) (177-230 μm in length by 5-7 μm in width, moderately motile) were screened in the wMicroTracker with 200 mf per well in 100 μL RPMI in both a 96-well U-bottom plate and a 96-well half-volume flat-bottom plate. Mf were screened with 18 replicate wells per treatment where the positive control was 50 μM ivermectin and the negative control was 1% DMSO. B. pahangi third-larval stage (L3) (1-2 mm in length by 26 μm in width, highly motile) were screened in the wMicroTracker with 10, 25, and 50 L3 per well in 200 μL RPMI in a 96-well U-bottom plate with 3replicate wells per condition. Schistosoma mansoni schistosomules (110 μm in length by 18 μm in width, not very motile) were screened in the wMicroTracker with 100, 200, and 300 schistosomules per well in 100 μL RPMI in a 96-well U-bottom plate with 8 replicate wells per condition. Trypanosoma cruzi epimastigotes (25.6 μm in length by 1.9 μm in width, moderately motile) were also screened in the wMicroTracker with 10,000, 50,000, and 100,000 epimastigotes per well in 100 μL DMEM in a 96-well U-bottom plate with 6 replicate wells per condition.
Based on the results of our screens with various parasites using the wMicroTracker and the WormAssay, it is recommended that the WormAssay be used to screen parasites greater than 10 mm in length and that the wMicroTracker and the modified WormAssay, the “Worminator”, be used to screen parasites less than 1 mm in length. When screening parasites in the wMicroTracker that do not travel throughout the well (e.g. schistosomules, B. pahangimf, B. pahangi L3) it is recommended to employ a 96-well U-bottom plate to ensure the parasites do not settle at the sides of the well and that they are able to cross the stationary LED beam at the center of the well. When screening parasites that are larger than C. elegans adults in the wMicroTracker, use fewer parasites per well and when screening parasites in the wMicroTracker that are smaller than C.elegans adults, use more parasites per well. Motility of Trypanosoma cruzi epimastigotes was not detected in thewMicroTracker. The aim is to detect mean movement units around 20-40 per well. We believe that these methods will help the research community develop better screening methods and shorten the time to optimize a screening platform.
MicroPubl Biol. 2020 Jul 20;2020:10.17912/micropub.biology.000279. doi: 10.17912/micropub.biology.000279.
Emma Gunderson , Christina Bulman , Mona Luo , Judy Sakanari
