Hardware specifications
What is the beam size relative to the worms?
The LED infrared microbeams have a diameter of 100-150 µm, which is similar to the width of an adult C. elegans worm.
What are the beam specs?
- Wavelength: Infrared, 880nm. low power (<1mW) no heat generated
- Whole MicroPlate scan frequency: 7 frames per second aprox
- Number of beams per well for the wMicrotracker ONE.
- 96F-well plate: 2 beams per well
- 96U-well plate: 1 beam per well
- 384-well plate: 1 beam per well
- Number of beams per well for the wMicrotracker MINI.
- 96F-well plate: 1 beam per well
- 96U-well plate: 1 beam per well
Those properties cannot be modified.
Do worms develop an aversion to the beam over time?
The infra-red beams in the wMicrotracker are generated by low-power LEDs and have been shown to be non-invasive for C. elegans (Simonetta and Golombek 2007).
Can I control/change the temperature of my samples with the wMicrotracker?
The temperature in the wMicrotracker cannot be pre-set and will depend on the room temperature. However, it is very compact and can easily fit in your incubator if needed.
The wMicrotracker does measure and report the temperature inside of the platform for the duration of the experiment. Note:
The wMicrotrackers power source does not generate heat.
Can I put the wMicrotracker inside an incubator?
When possible, make sure to reduce humidity in the incubator. When using humid chambers or very short experiments, use a maximum experimental timeline of 12 hours.
Can I put the wMicrotracker inside a CO2 incubator or hypoxia chamber?
Passive diffusion of the Gas, the instrument is permeable to gas, so you can use a CO2 incubator or hypoxia chamber when appropriate.
Do
the beams maintain the same properties overtime?
We observed no degradation of the LEDs over time. The LEDs in the wMicrotrackers have a lifetime of 36,000 hours. Since they flash every 1/384 sec, this is equivalent of 10 years of use.
The Microtracker was engineered to auto-compensate and auto-calibrate the beams to maintain signal linearity over time.
What are the computer requirements to use the wMicrotrackers?
Computer
minimum requirements:
– Pentium II processor or above (>1GHz
clock)
– 512Mb of RAM memory
– 1 USB port
– Windows XP
32 bits (or higher) operating system
– 200Mb of free HD space
(>10Gb free HD space recommended for real time data saving)
Experimental set-up
How many worms should I put in each well to get accurate measurements?
Larvae: For L1 to L3: use round-bottom 96-well plates – 100-150 larvae.
Older worms: For L4 and older worms in a flat bottom 96-well plates – 30-70 worms.
For more information on this matter please check out this Tech note.
Can I use bacteria in the well and still get accurate measurements of movement?
Yes. Worms can be measured in the wMicrotracker for multiple days when placed in bacteria. We recommend that you use fresh bacteria at an OD600 of 0.5 (up to 1). Under these conditions, adult worms usually have enough food for 3-5 days. Another option is to cultivate your worms in axenic media (CeMM) instead of using bacteria.
Is the wMicrotracker compatible with any worm strain?
The system is compatible with most worm strains including wild nematode isolates and parasitic nematodes. Because of the detecting methodology, non motile/non-good swimming strains (such as roll or severe unc mutants) are not recommended. Please refers to ARENA or SMART technology for unc applications.
How long can I keep and measure my worms in the wMicrotracker?
The wMicrotracker can acquire data on your worms for weeks without interruption.The limiting factor for longitudinal studies will be determined by your worm culture protocol and requirements. For long term data acquisition in the wMicrotracker, we recommend that you place your worms in axenic media. We recommend that you conduct measurements for at least 15 minutes to reduce standard error
What type of liquid media can I use?
96-wells experiments work with: liquid culture of E. coli OP50, CeMM, CeHR (with or without 10% skim milk). Which one to use will depend on your preference and your experimental requirements.
Note for lifespan experiments: it has been reported that liquid culture extend lifespan compared to NGM.
Should I use flat-bottom or round bottom multi-well plates?
The swimming activity of worms in U-bottom wells is generally higher than in flat bottom wells since the shape of the well causes the worms to accumulate exactly in the path of the laser beam which detects activity. In flat bottom wells, the nematodes are more spread out and interfere less often with the laser beam.
Do I need to count the worms in each well? How do I get good results if I can’t pipet precise number of worms in each well?
Pipetting worms is not as precise as dispensing same number of worms with a cell sorter or even manually. However, you can easily reduce the variability of your data by relativizing mathematically each group of wells to time 0 (referred to as “BASAL measure” in Simonetta et al.; 2007). In addition, check our pipetting protocol here.
Applications and data acquisition
What data do I get from the wMicrotracker?
The LED beams pulse every 1/384 sec and the software records every interruptions of the beams. This data is pooled and analyzed when the data report is generated by the software and presented as “average activity count by data interval”. You can always change the analysis bin (data interval) size after the data is collected. The minimum bin size recommended for analysis is 5 minutes. Choosing smaller bins may increase variability of your data.
What brands of plates can I use with the WMicrotracker?
The WMicrotracker was designed to fit multiwell plates from Greiner. Plates from other brands may not fit in the proper plate adapters.
For 384-well plates, you can use:
-384w COSTAR square shape,
-384w Microtiter round shape by Thermo Scientific
What is the size of the WMicrotracker files:
The WMicrotracker creates a folder for each experiment. The raw data for a 2-hour experiment is about 20 Kb. The Excel export file for such an experiment is about 15 Kb.
Can I perform worm development assays with a wMicrotracker?
Yes, the Microtracker can measure worm activity form L1 to older adult for weeks. Please make sure to validate your liquid culture protocol before using it in the Wmicrotracker.
Can I measure the worm’s curvature/body bends with the microtracker?
Although the wMicrotracker ONE does not have a camera, it is possible to correlate the wMicrotracker’s data (number of beam breaks) to the number of body bends per minute.
Can I measure movement in C. elegans larvae?
You can measure movement in populations of larvae as early as L1. Because of the small size of each worm, it is important to place enough worms in the well in order to generate a worm density high enough to disturb the beam.
Is it possible to measure individual worms?
It is not recommended (See graphs in this TechNote for more details).
Would V bottom be better in concentrating the worms than U bottom 96 well plates?
The V-bottom can work but using a U-bottom plate actually increases sensitivity, especially when using a low number of worms! We recommend filling the well to 40μl. We recommend using at least 25 adult C. elegans per well in a 96-well plate. If you’d like to learn more about experimental set-up and worm number, check out this tech note of the subject.
Can I change the measured intervals to be reported more than 30 minutes. Less than 30 minutes?
The Microtracker is constantly detecting and recording movement. When you export your data to an excel sheet, you can change the “Bin Size” drop down menu to the desired time interval whether it’s 8 minutes or 4 hours. The exported data sheet will then tell you the average activity count at the intervals you specified.
Does the wMicrotracker software recognize the source of activity (i.e. whether in one well 50 are dead but 1 very active worm. What about 25 dead, but 25 mildly active worms?)
The wMicrotracker utilizes 2 beams per well in a 96-well plate. So 1 very active worm shouldn’t be a problem because the activity score is an average per well. However this is when using a sufficient number of replicates becomes very useful to parse out the outliers.
Are the activity counts dependending on the size of the worms or only on their motility?
i.e. Is L1 or L4 will we have a lower/higher activity?
In a way, yes. You can measure movement in populations of larvae as early as L1. Because of the small size of each worm, it is important to place enough worms in the well in order to generate a worm density high enough to disturb the beam.
Larvae: For L1 to L3: use round-bottom 96-well plates – 100-150 larvae
Older worms: For L4 and older worms in a flat bottom 96-well plates – 30-70 worms
In this paper (figure 3A), the authors used the wMicrotracker to identify developmental stages. You can see the activity number increases as the worms grow. This is part due to the fact that the detection efficiency of the system increases as the worms grow.
Are you planning on comparing the activity number?
How long do you have to scan the plate in order to measure the activity of the worms?
30 minutes is the reccomended time lapse. However, you can measure worm activity at least for 10 minutes to ensure a reproducible dataset.
Is it possible to analyze worms growing on larger plates (30 or 60 mm diameter) with the wMicroTracker?
The wMicroTracker specializes in activity reading in liquid only and in multiwell plates.
Data analysis
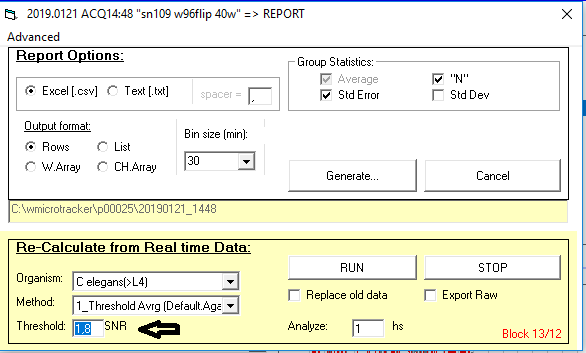
Which analysis method and threshold should I use?
We recommend that you use the standard setting for your data analysis:
(i.e. 1_Threshold Avrg; 1,8). This setting works for most applications.
Should we be using adult worms without eggs?
When measuring activity of gravid adults for a long period of time, it is important to consider the egg laying rate, as activity from a high number of L1s will be detected wy the wMicrotracker.
Can we put smaller worms like L4s. If we do, how many worms can we put per U-bottom well?
You can measure movement in populations of larvae as early as L1. Because of the small size of each worm, it is important to place enough worms in the well in order to generate a worm density high enough to disturb the beam.
- Larvae: For L1 to L3: use round-bottom 96-well plates – 100-150 larvae
- Older worms: For L4 and older worms in a flat bottom 96-well plates – 30-70 worms
Worms clog the pipette tips.
We usually use glass pipettes or disposable plastic pipette. What you can try is cut the tip of the pipette tip.
What numbers of adult C. elegans would you suggest to use when working in 384-well plates?
We suggest you use 15-25 adult N2s per well in a 384-well plate.
Would you recommend using less than 50 adult worms for 96 U-bottom plate.
The U-plate motion detection saturates more quickly than in flat bottom plates. We compared the results of a population of between 10 and 30 worms in this Tech note (see graph). We suggest you use 25-30 adults per well in a U-bottom 96-well plate.
Is it OK if some of the wells are empty without worms and fluid or do we have to fill in the 100 ul fluid in the empty wells.
It is OK to leave wells dry. However if you wish to use empty wells as negative controls/blanks, then you should fill them with the same buffer used for your experiments.
Note: The software will give you data for all the wells. You can just ignore the results in the dry wells. It is not uncommon for empty wells to return a non-zero, low activity value. It is usually due to the light refraction caused by the uneven well bottom. Adding buffer helps prevent this.
Troubleshooting
The worms stick to the plate. How can I fix it?
Some brands of plasticware can create more electrostatic issues. If your worms tend to stick to your plasticware when in M9, we recommend that you add 0.015% BSA, or 5% liquid NGM or 5% OP50 bacteria to the medium.
The temperature detected inside the Microtracker is different than the outside temperature. Is the WMicrotracker heating up?
we don’t see any heat using 9vdc power source).
When testing the temperature inside the unit using 9V power source, we did not observe any heating effect inside the WMicrotracker. The temperature sensor electronics is designed at 20ºC, there can be a 5% deviation at high temperatures (for example when placed inside an incubator).
We recommend that you complete a temperature Calibration using the following path: File / Advanced / Factory Settings.
We recommend that you use a mercury thermometer to calibrate against actual temperature.
How do I know which plate adapter to use for my experiments?
When selecting the plate of your choice on the software, you will be prompted to use the appropriate plate adapter. For 12-well plates, please use the 384 plate adapter.
In the set-up window, the blue dots which indicate the active laser points within a well are not evenly distributed between wells. Can I still compare the data between wells?
The
WMicrotracker was designed and build measurement of worm locomotion
in 96 and 384-well plates in mind. It is still possible for the users
to use other plates, however, the homogeneity of number of sensors
per well is not possible in such plates, due to the sensor/microbeam
hardware arrangement.
However, because the activity output is
calculated by averaging activity of all sensors within a well, these
differences in sensor numbers minimally affect the average activity
per well.
The threshold activity is not linear. What does this mean?
The threshold activity per well is calculated approximately as the 5% of the value of the signal. Since the signal changes as a function of the worms’ position and the OD of the culture medium, the average and therefore the threshold line also moves.
The activity plot shows an approximation f the activity and threshold. The applied threshold is recalculated every scanning round (i.e. every 500 samples).
The temperature inside the WMicrotracker is higher than room temperature.
Make sure that the WMicrotracker is not exposed to direct sunlight or near a source of heat.
Make sure that you are using the 9V DC power source adapter sent with your WMicrotracker.
I get an Error 6: Overflow when I try to analyze my data. Why?
What is the location of the installation folder?
Try moving this folder to the following location: c:\wmicrotracker.
My Microplates do not fit into the WMicrotracker adaptors.
Here is a list of the microplates that we tested in the Wmicrotracker:
384-well:
- 384w COSTAR square shape,
- 384w Microtiter round shape by Thermo Scientific
24-well, 96-well:
- Greiner
What if I am testing parasites, or need to keep my worms incubated at higher temperatures?
The Microtracker is calibrated to measure temperature at a range between 15°C – 35°C. If you plan on using the Microtracker outside of this temperature range, we recommend calibrating your machine by following these steps.
1. First, incubate and acclimate your Microtracker to the desired temperature.
2. Select ‘Run Latest Project from the main menu
3. At the top left corner, click ‘File and select ‘Advanced, then click on ‘Factory Settings
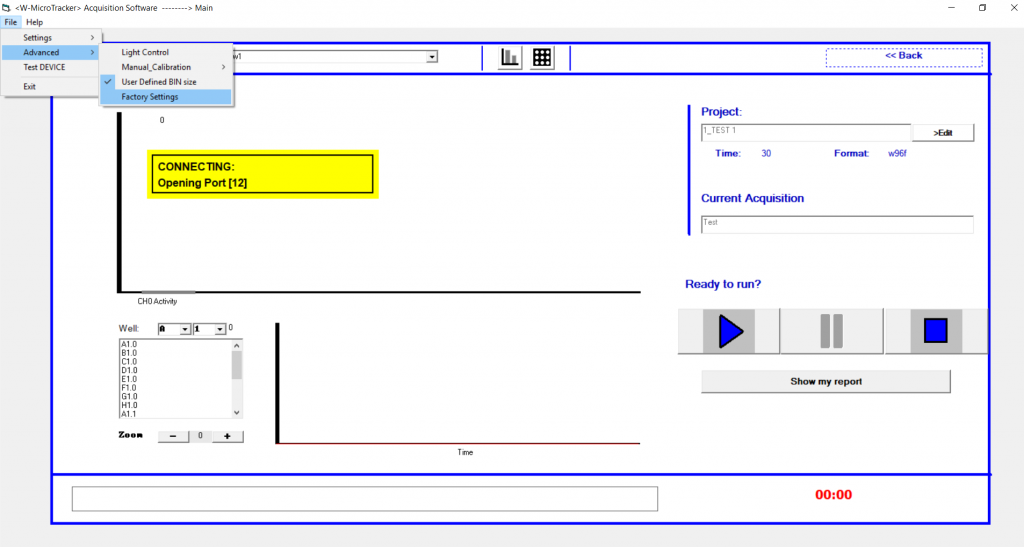
4. A pop up window will display the Current Value and Calibration T° input boxes.
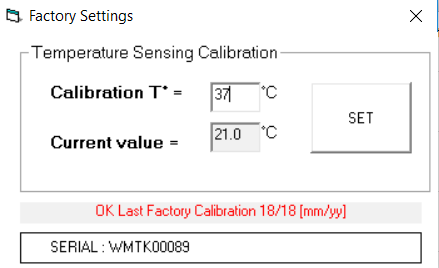
5. Confirm the incubated temperature of the Microtracker using a separate temperature gauge.
6. You can now change the Calibration T° field to your desired temperature. Select ‘SET’ and close the window.
Can I change the threshold if I am using a different model organism?
The default value of 1.8 is optimum for C.elegans and most nematodes detection.
However, it is still possible to reanalyze the data from recorded experiments utilizing the desired threshold value.
Note*: Backup your project before modifying your report with the specified threshold value.
1 ) From the main menu, select ‘Analyze recorded experiments’

Select your experiment (project/subproject) and double click to upload into the computer memory
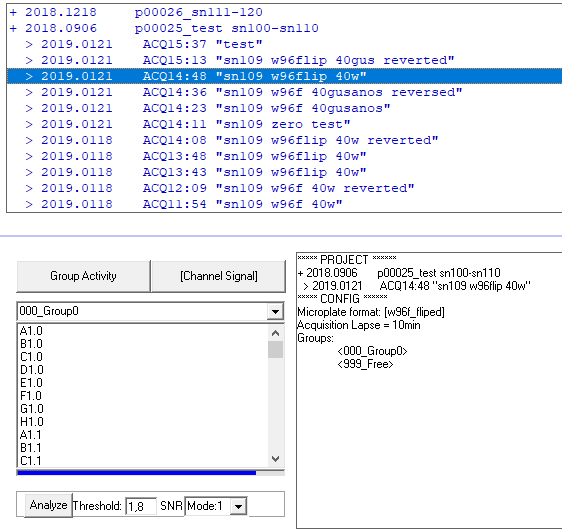
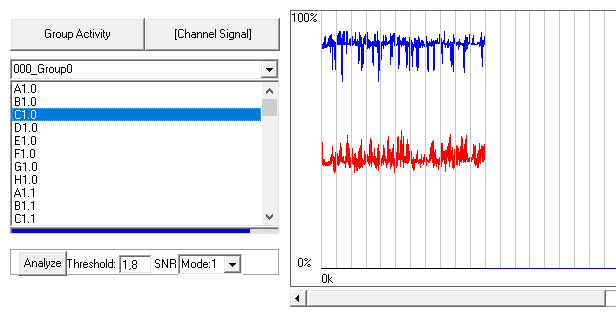
Plot raw data by pressing “Channel signal” and double click on any channel from your group
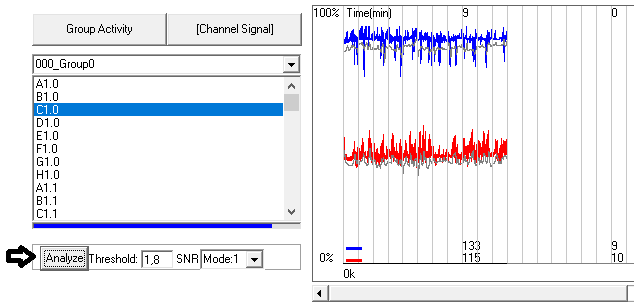
Check the threshold value most optimal for your data. Hit the “Analyze” button to apply and “Channel signal” to refresh the plot.
NOTE: check positive and negative controls in order to make sure the change is improving your detection.
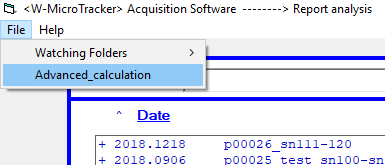
Once you have your desired threshold value, click on ‘File in the top left corner. Select ‘Advanced_calculation’, then hit ‘reanalyze raw data’
Select the required threshold to apply, unclick to “replace old data” and press RUN