An example of a toxicity test using our technology:
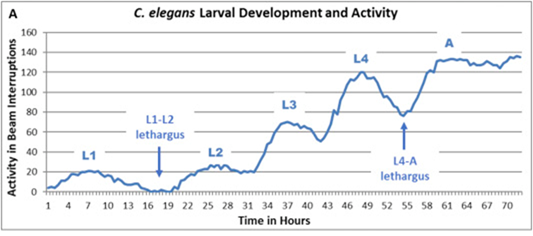
In “C.elegans Development and Activity Test detects mammalian developmental neurotoxins” (2018), Hunt et al describes a novel assay for developmental neurotoxicity (DNT) that measures both developmental timing and activity levels in C.elegans larvae, the worm Development and Activity Test (wDAT). C.elegans larval development progresses through four distinct stages that can be identified by larval size and morphology. Between each of these stages, the developing worm enters a short period of lethargus during which the cuticle is shed and locomotion and feeding slow or stop (Figure 1).
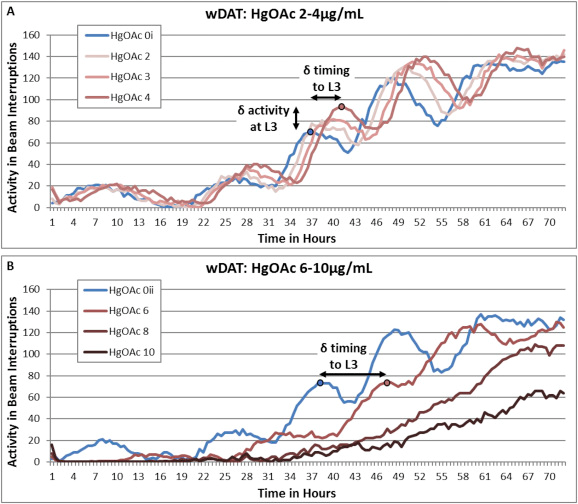
Using WMicrotracker One, Hunt et al. were able to perform an automated assessment of periodic levels of activity and inactivity with the potential to map C.elegans developmental progress through the four larval stages and into adulthood. Changes in wDAT activity peak height relative to control would indicate stage specific altered motor activity levels for dosed populations. The wDAT was able to detect developmental delay and hyperactivity for arsenic, lead, and mercury (figure 2), heavy metals that are known human developmental neurotoxins and have been associated with hyperactivity in children.
Good C.elegans Culture Practice:
A review article in Journal of Applied Toxicology by Piper Hunt from Division of Applied Toxicology (FDA-USA) highlights the utility of the C.elegans model for screening at an early step in predictive toxicity testing. The article summarizes the strength and limitations of the model organism and also describe good C.elegans culture practice (GCeCP) essential to ensure reliable and repeatable results in C.elegans toxicity assays.
“Caenorhabditis elegans is a small nematode that can be maintained at low cost and handled using standard in vitro techniques. Unlike toxicity testing using cell cultures, C.elegans toxicity assays provide data from a whole animal with intact and metabolically active digestive, reproductive, endocrine, sensory and neuromuscular systems. Toxicity ranking screens in C.elegans have repeatedly been shown to be as predictive of rat LD50 ranking as mouse LD50 ranking. Additionally, many instances of conservation of mode of toxic action have been noted between C.elegans and mammals. These consistent correlations make the case for inclusion of C.elegans assays in early safety testing and as one component in tiered or integrated toxicity testing strategies, but do not indicate that nematodes alone can replace data from mammals for hazard evaluation.”
According to the review article, “C.elegans used in toxicity testing must be maintained in a highly consistent manner, with temperature, salt concentration and sufficient nutrient supply held constant to ensure reliable, repeatable results (Table 1). Alterations of even a short duration in any of these variables will lead to altered gene expression and toxin response, potentially for multiple generations.”
Table 1. C.elegans culture standardization factors for toxicology and GCeCP. (Adapted from The C.elegans model in toxicity testing – Piper Reid Hunt).
| Factor | Details |
| Temperature | Small temperature differences have a large effect on C.elegans growth rate, motility, lifecycle, lifespan, and gene expression. |
| Humidity | Low humidity will alter test article and nutrient concentration, the smaller the volume the larger the effect. |
| pH | Extreme pH is required to alter adult C.elegans viability, but other endpoints are more sensitive to pH. The appropriate pH range should be determined for each assay. |
| Worm Density | C.elegans gene expression and life cycle respond to nutrient availability and secreted hormones. |
| Cohort Synchronization | L1 C.elegans can be isolated by hypochlorite treatment of gravid hermaphrodites followed by hatching of the released eggs in non-nutrient buffer. After more than 18 hours in the absence of nutrients, gene expression is altered resulting in delayed and unsynchronized development, and increased stress resistance. |
| Dauers | C.elegans dauers secrete dauer pheromone, which promotes conversion to the dauer state in other larvae and induces increased stress resistance in exposed adults. This will both reduce apparent growth rates as measured by worm length, and increase viability in the presence of many toxins. |
| Genetic Drift | If C. elegans cultures are consistently well fed for optimal toxicity studies, use of frozen stocks must be scheduled in order to prevent genetic drift. |
| Males | Non-disjunction of the X chromosome results in XO males. This happens rarely in nature, and is induced by toxins and stress. Males are smaller than the XX hermaphrodites, which will result in apparent reduced growth. Mating with males more than doubles the progeny per hermaphrodite relative to selfing, so males in a culture will increase reproductive output. |
| Solid vs. Liquid Medium | When the test article is mixed into molten agar, the true exposure will depend on many factors. Dosing in liquid medium provides a measureable exposure, but limits the test to water-soluble compounds. |
| E. coli vs Axenic medium | Axenic medium avoids the complicating factor of the metabolic response of the feeder organism. |
Hunt PR (2017)The C.elegans model in toxicity testing. J. Appl. Toxicol. 37:50–59.
Hunt PR, Olejnik N, Bailey KD, Vaught CA, Sprando RL (2018) C.elegans development and activity test detects mammalian developmental neurotoxins. Food Chem Toxicol 121:583–592
